Details of the Drug
General Information of Drug (ID: DMJ8NMC)
| Drug Name |
Barbital
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Barbitale; Barbitalum; Barbitone; Barbitonum; DEBA; Diemal; Diemalum; Diethylbarbitone; Diethylmalonylurea; Dormileno; Dormonal; Ethylbarbital; Hypnogene; Malonal; Medinal; Sedeval; Uronal; Veroletten; Verolettin; Veronal; Vesperal; Barbital Faes Brand; Barbitale [DCIT]; Diethylbarbituric acid; Faes Brand of Barbital; Barbital (TN); Barbital (VAN); Barbital [INN:JAN]; Barbitalum [INN-Latin]; Veronal (TN); Barbital (JP15/INN); Kyselina 5,5-diethylbarbiturova; Kyselina 5,5-diethylbarbiturova [Czech]; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5,5-diethyl-(9CI); 5,5-Diethyl-2,4,6(1H,3H,5H)-pyrimidinetrione; 5,5-Diethylbarbituric acid; 5,5-diethyl-1,3-diazinane-2,4,6-trione; 5,5-diethylpyrimidine-2,4,6(1H,3H,5H)-trione; 7979P
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
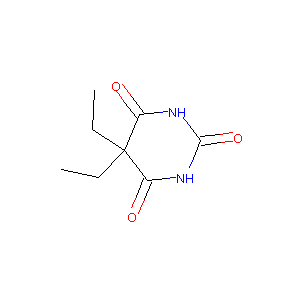 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 184.19 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
